 |
| cool beard |
Now on to the actual periodic table. There are 118 elements on it. Yeah, I know, that's a lot of elements. But, elements over 92 are too unstable to be made in nature so it has to be made in a lab. If you guys were wondering what 92 is for, then I'm about to tell you. If you weren't thinking that, then plug your ears because you're not allowed to hear! Just kidding! The "92" is the elements atomic number. That shows how many protons are in their atomic nuclei. The atomic number is at the top and under that is the elements symbol. The symbol is the 2 or 3 letter abbreviation for the element. Like Helium would be He. Under the symbol is the name of the element. And finally, under that is something called the atomic mass. That is exactly what you'd expect it to be, the mass of an atom. Basically, it's how much the atom weighs. Since most of the mass of an atom is in its nucleus, it's technically how much the nucleus of an atom weighs. Here's a picture of how an element looks on the periodic table:
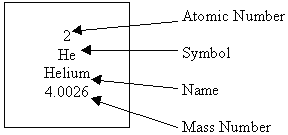 |
| see, this is how Helium looks |
Elements are put in order from there atomic number. They are also put in these things called groups and periods. Groups are elements with similar configurations of electrons. They are put in vertical columns and are numbered 1-18. Periods describe the number of electon shells that have elements have. Do you remember learning about electron shells? They are what electrons orbit on. Well, each element gets an electron added to thier shell. So the first one, starting from the left, has one on it's outer shell, and the one on the other end, the right side, is completely full. Sorry for confusing you all but hey it's not MY fault your brains don't process this information!! Haha just kidding!!

Another thing you should know are catagories. These are elements that have the some properties. There are 7 different groups. Alkali metals, alkaline earth metals, transition metals, poor metals, semimetals, nonmetals, and noble gases.
That my friends, was the periodic table. I hope you liked!! Ok, now on to our next topic. Oh you thought the blog was over????????
IT IS! Science Fun:)
 |
| YOU THOUGHT THE BLOG WASN'T OVER |
lol the tripod pic was supposed to b how helium looks on the periodic table in case u were wondering
ReplyDelete